Cancer remains the number 2 killer in the United States, causing 22% of all deaths (just behind heart disease which is at 23%). The good news, however, is that the overall cancer incidence has been stable in women and declining steadily in men, and our ability to treat various types of cancer keeps improving.
As all aspects of cancer research and treatment evolve, cancer incidence should start to decline significantly regardless of gender, and our ability to treat every type of cancer will improve incrementally. However, to get to this point, we must find the answers to many complex questions regarding cancer.
Fortunately, research has been uncovering the many mysteries of cancer for decades, revealing clues to how we can keep cancerous cells from developing and growing with a combination of lifestyle/dietary changes and targeted forms of standard cancer therapies.
One nutritional therapy that is picking up momentum both in the research and oncology communities is the ketogenic diet. While this is not a cure for all cancers, there are many cancers that are responsive to this dietary change.
Later in this article, we will explore the research behind the keto diet and cancer and how it applies to you and your loved ones — but first, let’s develop a deeper understanding of cancer.
What We Know About Cancer So Far
There are over 100 documented forms of cancer, and the five most common worldwide are lung, breast, colorectal, prostate, and stomach. The one thing that each one of these cancers have in common is uncontrolled cell growth.
To carry out their growing process, cancer cells will typically:
- Avoid apoptosis (programmed cell death)
- Decrease their resistance to growth
- Rely on a yeast-like energy metabolism and growing process
- Evolve quickly and adapt to their environment with each iteration
- Stimulate increased vascularity and blood flow to feed itself
- Invade local tissues and spread to other tissues through the blood
- Exchange genetic material with other cells to improve evolving capacity
All cancers won’t exhibit each one of these traits, but these are the traits responsible for the insidious, mysterious nature of many cancer cells.
These abnormal cell processes are initiated as a result of genetic alterations that lead to abnormal cell proliferation. The cancer cells will then typically form a lump called a neoplasm. People commonly call neoplasms “tumors.”
Scientists refer to tumors that do not spread to other parts of the body as “benign tumors.” These are not cancerous. Tumors that do spread to other parts of the body are cancerous and called “malignant tumors.”
Whether a tumor is benign or not depends on the environment that they are in and the genetic changes that take place to those cells. For example, some mutated cells may be limited by their genetics and environment to the point that they stop growing, don’t spread into other tissues, and become benign tumors.
On the other hand, other mutated cells can stimulate increased blood vessel growth and develop more growing capacity, allowing them to divert more resources to themselves and proliferate with less resistance. Eventually — if the growth continues — these cells will become known as “malignant tumors”.
Fortunately, the development of cancer is a multistep process in which cells gradually become malignant through a progressive series of alterations that may take years to decades. This gives us the opportunity to catch cancers at earlier stages and treat them before they evolve, become stronger, and spread throughout the body.
In fact, we may even be able to stop cancer in its tracks before it even becomes a neoplasm. The key to figuring out how we can do this is by understanding what causes cells to become cancerous.
What is the Cause of Cancer?
Since the development of cancer is a complex multistep process, many factors may affect the likelihood that it will develop, and it is overly simplistic to speak of single causes of most cancers.
Nonetheless, many agents, including radiation, chemicals, and viruses, have been found to induce cancer in both experimental animals and humans.
The factors that seem to initiate cancer are called initiating agents, and they cause cancer by damaging DNA in a way that can lead to cancer-provoking mutations. Some of the initiating agents that contribute to human cancers include:
- Solar ultraviolet radiation (the major cause of skin cancer)
- Ionizing radiation (most common sources are radon and X-rays)
- Carcinogenic chemicals in tobacco smoke (the undisputed cause of 80 to 90% of upper respiratory tract and lung cancers)
- Carcinogenic chemicals in smoked, grilled, and/or charred meats (e.g., heterocyclic amines and polycyclic aromatic hydrocarbons)
- Certain viruses (commonly responsible for liver cancer, cervical carcinoma, and cancer-causing mitochondrial dysfunction)
- Aflatoxin (a potent liver carcinogen produced by some molds that contaminate improperly stored supplies of peanuts and other grains)
Other compounds can contribute to cancer development by stimulating cell proliferation without causing DNA damage. These compounds are referred to as tumor promoters since the increased cell division they induce is required during early stages of tumor development.
Hormones, particularly estrogens, play a crucial role as tumor promoters in the development of some human cancers. The link between estrogen and cancer is a major reason why women have a higher risk of breast cancer than men. High estrogen levels are associated with an increased risk of endometrial and ovarian cancer as well.
In addition to initiating agents and hormones, obesity is emerging as a leading cause of cancer as well. This is due to the fact that having excessive amounts of fat tissue will:
- Increase chronic levels of inflammation which can damage our DNA and mitochondria in a way that can cause cancer.
- Lead to excess amounts of estrogen production, promoting tumor growth.
- Keep the levels of other growth-promoting hormones like insulin and IGF-1 high.
- Impair the healthy regulation of cell growth.
Overall, cancer is a complex disease with many causes and contributors, but it is becoming less mysterious and more understandable as more research is done. Cancer can develop multiple traits that make it more and more resilient, but we also keep making more and more discoveries that provide us with clues as to how we can prevent, manage, and treat various types of cancer — even those that are the most sinister.
One of these discoveries, in particular, gives us a better idea as to how we can change our diets and lifestyles to decrease our risk of cancer and improve the effectiveness of cancer treatment.
The Metabolic Link With Cancer — Give Me Some Sugar!
One of the first observations about the disease was that cancer cells seem to have an altered energy metabolism that relies primarily on glycolysis and glucose. These findings were largely made through experiments by the German scientist, Otto Warburg, in the 1920s.
He found that the tumor cells he studied would convert high amounts of glucose into energy and a byproduct of glycolysis called lactic acid. This is now known as the Warburg Effect.
However, Otto Warburg extended his observations a bit too far. He thought that the Warburg Effect was the cause of cancer. The current literature, however, reflects a different story, a story that consists of genetic mutations being the primary cause of cancer.
Nonetheless, altered energy metabolism is the one biochemical feature that the majority of cancer cells seem to share. According to the bulk of the evidence, the Warburg Effect “is an established hallmark of cancer.”
Studies in molecular models show that high levels of glucose are associated with increased cancer growth while depriving cancer cells of glucose seems to lead to apoptosis (programmed cell death). Epidemiological studies also show that high blood glucose, known as hyperglycemia, is strongly associated with increased risk of cancer.
The medical community has known about cancer cell’s preference for glucose for quite some time. In fact, one of the ways they get an image of a tumor is essentially by injecting a glucose-based “dye” into the body and using some sort of machine to see that “dye.” The area that lights up the most when taking the image is where the cancer tumor is – that’s because of the cancer cell’s overwhelming desire for glucose.
As you’ve looked into the keto diet, you’ve probably read that sugar is our primary fuel source, and this is true — but cancer cells handle glucose a bit differently. At rest, for example, our healthy cells will not produce lactic acid. Conversely, cancer cells have such an issue with normal energy metabolism that it essentially can only burn glucose in a way that produces lactic acid. By producing energy in this way, the cell will become more and more cancerous as it makes itself vulnerable to further mutations without any hope of repair.
In other words, cancer cells seem to regress into a cell that resembles yeast in the way it gobbles up glucose and grows gratuitously. It doesn’t care about what the other healthy cells are doing or about what the body wants because it is mutated to the point that it becomes its own self-serving entity.
Mutated Mitochondria — A Potential Cause of Cancer that We Can Address
All of this talk about energy metabolism leads us to another important finding in the cancer literature: healthy cells can also become cancerous if their mitochondria (the primary energy producer of the cell) is dysfunctional for an extended period of time. Furthermore, if the cell is already cancerous, the mitochondrial dysfunction will make the cell’s genes vulnerable to additional mutations.
As a result, these cells will need more and more sugar to survive, reverting further and further into yeast-like metabolism and growth.
Given this relationship between glucose, mitochondria, and cancer cells, an important question arises — Can a carbohydrate-restricted diet, such as the ketogenic diet, help with cancer because of how it limits sugar intake?
The Keto Diet and Cancer — Some Limitations From a Mechanistic Perspective
Theoretically, if most cancer cells thrive off of sugar, then restricting carbs with a keto diet should be enough to prevent cancer and/or induce apoptosis of cancer cells, right?
Unfortunately, there are some caveats with cancer that we must explore before we hop on the keto for cancer bandwagon.
The first caveat is that not every cancer is the same. Each cancer will accumulate different genetic mutations that will dictate how it survives, grows, and spreads. For example, some cancers may be able to fuel themselves with substrates other than glucose.
A recent research review stated that:
Besides glucose, glutamine can also serve as a major energy metabolite for some cancers. Glutamine is often present in high concentrations in culture media and serum. Cell viability and growth can be maintained from energy generated through substrate level phosphorylation in the TCA cycle using glutamine as a substrate.
In other words, an amino acid that you get from the protein-rich foods that are commonly eaten while keto dieting can be used to fuel certain cancer cells.
Two other caveats that are important to address are: (1) protein can be converted into glucose and (2) the consumption of other amino acids like leucine can stimulate cell growth.
When we restrict our carb consumption, our bodies still need sugar to function (even when we are in ketosis). To meet its sugar needs, the body will create sugar from non-carbohydrate substrates using gluconeogenesis.
Gluconeogenesis is the metabolic process by which the liver converts gluconeogenic amino acids (from protein) and glycerol (from stored fat) into sugar as a way to fuel the cells that need it. Unfortunately, this glucose can also fuel cancer cells and may diminish some of the beneficial effects that the keto diet can have for cancer patients.
Protein consumption can also stimulate a little protein present in the body called mTOR (short for mammalian target of rapamycin) which helps regulate cell growth, proliferation, survival, and protein synthesis (among other things). Something as simple as a big juicy steak will cause our body to upregulate (stimulate the increase of) mTOR — and when this happens, the cells can “forget” to do some very important things (like controlling and preventing cancer growth).
With all of these caveats considered, the keto diet may not be the cancer cure that some low carb lovers make it out to be, but it is still a great diet option that many cancer patients would benefit from in conjunction with their cancer treatment.
In fact, some researchers believe that the keto diet may be one of the best diets for preventing the growth of most cancers — especially those that are linked to obesity. This is because restricting carbs is a simple and effective way to improve mitochondrial function and protect our cells from the damage and inflammation that can lead to genetic mutation.
However, we must keep in mind that our assumptions are only mechanistically based at this point. To get a better idea of how the keto diet helps with cancer, we must look at studies that implemented the keto diet with cancer patients.
The Ketogenic Diet and Cancer — A Closer Look at The Research
The first study looking at the ketogenic diet and cancer was published in 1995 in the Journal of the American College of Nutrition. In it, researchers recruited two young female patients at the University Hospitals of Cleveland. Both had Glioblastoma Multiforme (GBM), a form of cancer that starts in the brain and rapidly spreads throughout the body.
GBM is the most aggressive cancer of the brain and is difficult to treat by using the conventional therapy of radiation and chemotherapy. The median survival of people with GMB with intensive, standard treatment is an average of 15 months. Both patients in this case study also had advanced stage brain tumors called “astrocytomas” that were unresponsive to conventional treatments.
As an intervention, the researchers asked the patients to consume a ketogenic diet for eight weeks that consisted of 60% medium chain triglycerides (MCTs). MCTs are a type of saturated fat that are digested rapidly and converted into ketones in the liver.
In both patients, levels of blood glucose decreased to low/normal levels and ketones increased by 20 to 30 times within seven days of starting the ketogenic diet. Results from scans indicated that there was a 21.8% decrease in glucose uptake at the tumor sites in both subjects. Lower glucose uptake is a strong indicator that a tumor is shrinking in size.
One patient experienced significant improvements in clinical health and overall mood throughout the study. She then continued the ketogenic diet for another year, and her disease did not progress at all.
Due to these findings, the authors concluded:
While this diet does not replace conventional antineoplastic treatments, these preliminary results suggest a potential for clinical application which merits further research.
A 2010 case study published in Nutrition & Metabolism also suggests that the ketogenic diet may help certain individuals with cancer. For their evaluation, researchers chose a 65-year old woman with GBM.
This particular patient had a tumor in her right hemisphere that led to chronic headaches, frequent fatigue, and increasing memory loss. Doctors removed some of the tumor through an incomplete surgical resection, but her symptoms persisted.
To control her tumor, the patient implemented a ketogenic diet for two months. Her dietary approach consisted of a water fast followed by a restricted 4 to 1 ratio diet of fats to carbohydrates and protein. Overall, this diet contained a total of 600 calories per day.
She supplemented the diet with minerals and vitamins to avoid micronutrient deficiencies. The patient also participated in conventional therapies to treat the tumor but stopped taking her steroid medications.
After two months, the patient had no detectable brain tumor tissue. A blood analysis revealed that she had lower levels of blood glucose and increased levels of urinary ketones. She also had lost 20% of her body weight, which was a desirable outcome in this case.
The patient experienced no notable side effects due to her ketogenic diet. After suspending her diet and therapy for ten weeks, her tumor resurfaced. The doctors reinitiated chemotherapy. She eventually succumbed to her illness less than two years after the initial diagnosis.
With this case study, it is important to note that patients with GBM rarely experience a rapid tumor regression after surgical resection and conventional therapy. Thus, the researchers emphasized that the “…response of the GBM in this patient after standard treatment alone would be unlikely, further suggesting a role for targeting energy metabolism as part of the management strategy.”
In their conclusions, they stated that the favorable response could be attributed “in part” to the calorie-restricted ketogenic diet. However, the researchers emphasized that “further studies are needed to evaluate the efficacy of restricted ketogenic diets, administered alone or together with standard treatment, as a therapy for GBM and possibly other malignant brain tumors.”
Key Takeaways: Case studies on patients with advanced cancer suggest that the ketogenic diet has the potential to slow down cancer growth when coupled with conventional treatments.
Digging Deeper with Pilot Randomized Trials
Researchers have not conducted formal, randomized clinical control trials on the ketogenic diet and cancer. Medical professionals and scientists regard these as the most rigorous method for assessing the efficacy of a clinical intervention, whether it is a prescription drug, diet, or other treatment.
However, researchers have conducted some pilot trials about how the ketogenic diet affects cancer. Scientists define these as:
…exploratory studies limited in size and scope that give insight into the actions, efficacy, and safety of a drug or device but cannot provide definitive support for specific mechanistic or therapeutic claims.
This means that pilot studies are smaller in scale than a standard clinical trial, but they still yield important evidence and indicate which treatments should be assessed further. Typically, scientists perform pilot studies after case studies and animal studies yield promising results, which is exactly what was done regarding the keto diet and cancer.
Scientists published the first pilot study on the relationship between the ketogenic diet and cancer in 2011. For their study, they recruited 16 patients (12 women and 4 men) with various cancers in advanced stages. They had an average age of 50.4 years (30-65 years). All of them had metastatic tumors, which means that they grew and spread throughout the body.
Researchers instructed the patients to follow a ketogenic diet for 3 months consisting of less than 70 grams of carbohydrates per day.
They asked the subjects to eat a protein/fat shake each day along with a diet rich in foods such as sunflower seeds, flaxseed, cold-water fish, high fiber vegetables, and organic red meat. Typically, dietitians regard these as healthy, low-carb foods.
Each meal contained no more than 20 grams of carbohydrates. The researchers monitored key blood parameters such as glucose and quality of life measures at the beginning of the study and then every two weeks until the study finished three months later.
Of the 16 patients, five stuck with keto for the full duration of the trial. Most of the patients who stopped did so for unrelated reasons, such as strong progression of cancer not associated with the ketogenic diet or death. Three patients had difficulty complying with the diet and stopped.
Tumors did not progress at all at all in the five patients that successfully completed the ketogenic trial. This is a positive outcome given the advanced stage of their cancer. Additionally, some of these patients experienced favorable changes in glucose, HDL:LDL ratio, triglycerides, and healthy levels of weight-loss. These findings further support the healthy impact a ketogenic diet may have on cancer.
Two patients also had significantly lower levels of CRP, a key indicator of inflammation, while the other three had relatively little change. This indicates that some patients who complete the diet may have more favorable responses than other patients may in regards to some parameters.
Many patients experienced worsening cognitive function and symptoms such as pain and fatigue throughout the trial. However, the scientists attributed this primarily to the advanced stage of the disease.
Because of their findings, researchers concluded that “these pilot data suggest that a KD is suitable for even advanced cancer patients.” They also noted that the ketogenic diet “has no severe side effects and might improve aspects of quality of life and blood parameters in some patients with advanced metastatic tumors.”
It is important to emphasize, however, that the ketogenic diet had a variable response. Some patients were able to comply with it better than other patients were. Additionally, of those that completed the trial, some had changes that are more favorable in certain parameters such as CRP. This suggests that the ketogenic diet is not suitable for everyone.
Another study from 2014 suggests that the ketogenic diet is a safe, effective treatment to manage aggressive cancers when used with conventional therapies. For their retrospective study, researchers reviewed 53 patients with glioma, a tumor that starts in glial cells in the brain and spinal cord. These patients were treated with chemoradiotherapy and other standard treatments from August 2010 to August 2013. Six of these patients also consumed a ketogenic diet while on treatment.
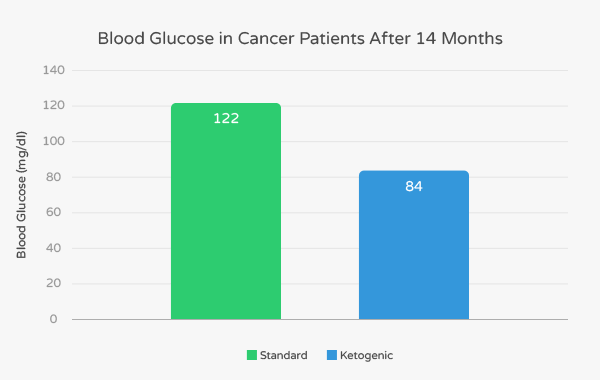
The ketogenic diet subjects tolerated the diet and experienced no toxicity; one did experience mild fatigue. At the follow-up of 14 months, four of these six patients were alive. The mean blood glucose of patients eating standard diet was 122 mg/dl (scientists define diabetes as blood glucose greater than 125 mg/dl).
In contrast, patients eating the ketogenic diet had an average blood glucose of 84 mg/dl. These lower levels of blood glucose are associated with more restrained growth of tumors and better management of cancer symptoms.
Due to these findings, the researchers stated:
Based on this retrospective study, a KD appears safe and well tolerated during the standard treatment of GBM.
They also noted that the ketogenic diet reduces blood glucose even when used with steroids such as Dexamethasone that treat tumor swelling.
Often, a negative side effect of steroids is higher blood sugar. The researchers also noted, “Larger prospective trials to confirm this relationship are warranted.”
Key Takeaways: Pilot trials in cancer patients indicate that the ketogenic is safe and effective in some patients when used with standard treatments. More research needs to be done to further assess the value of carbohydrate restriction in treating symptoms of cancer.
Putting the Research in Context
What does the available literature as a whole say about carbohydrate restriction and cancer? A meta-analysis by Schwartz et al. looked at the literature on 32 glioma patients treated using the ketogenic diet as an alternative or complementary therapy.
All of these patients had measurable disease after going through conventional therapies. Their life expectancy was at least three months.
Six of these patients were from the study above by Champ et. al. Five were from case studies including the three from the studies by Nebeling et al. and Zucculo et al. discussed above.
Nineteen of these patients were from a randomized pilot study conducted in Germany by Rieger et al. and two of them were case studies enrolled by the authors of the meta-analysis.
Researchers discovered that the ketogenic diet yielded no major side effects in patients. However, some patients did not personally like the diet and had difficulty sticking with it. The authors suggested that regular support from a dietitian or nutrition community (such as Ruled.me) could improve compliance.
They also noted that some patients were more responsive to the ketogenic diet than other patients were. The best response was in a 3-year-old girl who had complete remission five years of treatment with a ketogenic diet. Two other patients also experienced complete remission after the diet, and the other two patients had disease progression after stopping keto. (Keep in mind, however, that all of these patients used conventional treatments along with the keto diet.)
The two patients the authors of the meta-analysis treated with the ketogenic diet had disease progression. They attributed this in part to the lack of regular contact these patients had with a dietitian.
In the large pilot study by Rieger et al., they noted a “trend towards an increase in progression-free survival was reported in patients with stable ketosis.” They noted that a dietitian did not support subjects and that steroid drugs may have increased levels of blood glucose.
The authors of the study concluded that the ketogenic diet “is safe and without major side effects… treatment with KD may be effective in controlling the progression of some gliomas.” They emphasized the importance of working extensively with a dietitian to adjust the patient’s diet as needed and measure ketones and glucose.
They also suggested that a keto-friendly community (such as Ruled.me) might be useful service in ensuring that people stick with the diet. Additionally, they commented on the need for more rigorous research, stating that:
“Further studies are needed to determine factors that influence the effectiveness of KD, whether as a monotherapy or as adjunctive or supplemental therapy in treating glioma patients.”
Back to the Biochemistry: How Does Keto Combat Cancer?

The preliminary evidence behind using the ketogenic diet to treat cancer as a complementary treatment to conventional therapies is promising. Let’s recap how scientific theory explains the findings.
Four main mechanisms seem to be responsible for the keto diet’s ability to combat cancer:
- Energy Deprivation. By its nature, the ketogenic diet is very low in carbohydrates (typically 20 to 50 grams/day) and naturally restricts calorie consumption. This restricts the amount of fuel that cancer cells receive, even for the cancer cells that are able to thrive off of multiple substrates. Furthermore, almost all cancer cells seem to lack the ability to use the ketones produced when carbs consumption is restricted. Thus, cancer patients who are keto-adapted will probably be the most effective at starving cancer cells.
- Growth Factor Suppression. The ketogenic diet suppresses insulin-like growth factor (IGF-1). This molecule is associated with the formation and progression of cancerous cells. It is “upregulated” when you eat more carbohydrates, making it more likely to trigger cancer growth. Because the ketogenic diet is much lower in carbohydrates, scientists suspect that this suppresses IGF-1 production. This ultimately slows the formation of cancerous cells.
- Increased Mitochondrial Efficiency. Burning fat and ketones for fuel is so efficient that the process helps boost mitochondrial function and decrease the production of free radicals that can lead to inflammation and cell damage. This can prevent mitochondrial dysfunction from causing and feeding cancer growth.
- Apoptosis Induction. Studies show that dietary energy restriction enhances phosphorylation of adenosine monophosphate kinase (AMPK), which has been found to induce apoptosis in glycolytic-dependent brain cells and protect normal brain cells from death. One way to naturally restrict energy consumption is with a keto diet because most keto dieters spontaneously eat fewer calories than they do when they are on a higher carb diet. Altogether, this may explain why most of the research on keto and cancer has shown the keto diet to be effective in the treatment of brain tumors (glioblastomas and gliomas).
Despite how effective the keto diet seems to be for cancer, we must remember that this is only for certain types of cancer. Gliomas and glioblastomas, for example, tend to have dysfunctional mitochondria and rely on sugar more than any other types of cancer.
Will the keto diet be as effective for other types of cancer as well? Additional studies have shown that ketogenic diets reduce tumor growth and improve survival in animal models of colon cancer, gastric cancer, and prostate cancer as well — but the research doesn’t provide us with a clear answer for the treatment of these cancers in humans.
Unfortunately, the scientific community has yet to find reliable ways to identify the nature of different cancers so that we can know for certain if a keto diet is indicated for specific cancer patients. For this reason, it is best to make dietary changes in conjunction with standard cancer treatments. Treating cancer with diet alone is risky and not recommended at this point.
Arguably the best cancer treatment plan should include lifestyle and dietary adjustments that help the patient prevent cancer growth and improve overall health along with the appropriate traditional cancer therapies (radiation therapy, chemotherapy, surgery, etc.).
For more information on the lifestyle and diet changes that you can make to reduce the risk of cancer and potentially stop cancer growth, continue reading the rest of the article.
More Diet and Lifestyle Changes that Can Prevent Cancer Growth
Below, we’ll explore different changes in lifestyle and diet that can prevent or slow the growth of cancer.
Intermittent Fasting
Intermittent fasting is a common practice used to boost weight loss, but did you know that it is effective at preventing cancer growth as well? While we are in a fasted state, our normal cells simply flip a metabolic switch, increasing ketone production and fat burning and reducing sugar use.
Conversely, most cancer cells don’t have this ability.
As a result, these cells will struggle to survive because the fuel available is not the fuel they can use. This will essentially starve these cancer cells (albeit slowly since intermittent fasting is not a constant fasting state – but, with the “always on” state of a cancer cell, even short, intermittent fasts, will slow and starve it).
The research on how extended intermittent fasts affect cancer patients backs up our biochemical understanding as well. In initial case studies, cancer patients who were undergoing chemotherapy voluntarily fasted for anywhere between 48 to 140 hours (much longer than the intermittent fasts that keto dieters typically do). Each person reported fewer side effects and an improved quality of life regardless of how long they fasted.
This implies that fasting for 2 days to a week can have a protective effect on the body while it is undergoing intense bouts of toxicity from cancer treatment.
Other studies have found that fasting was as effective as chemotherapeutic agents in delaying progression of different tumors.
Although this research may not apply directly to your life, it does suggest that different variations of intermittent fasting can help support your body in times of toxic stress, whether it is during chemotherapy or after a cheat meal.
Calorie Restriction
Calorie restriction, while more difficult than intermittent fasting for some, has shown promising results in preventing and starving cancer for the same reasons as intermittent fasting. Basically, calorie restriction will cause cancer to run itself out of fuel because of its constant need for glucose and lack of metabolic flexibility. Once that happens, the cancer may begin to starve and die off.
Additionally, restricting your calorie intake will positively impact your baseline blood glucose levels, reduce inflammation, and improve overall mitochondrial health, all of which will reduce your risk of cancer, prevent healthy cells from becoming cancerous, and keep cancer cells from reproducing.
So far the research has found energy restriction to significantly reduce growth and progression of numerous cancers including mammary, brain, colon, pancreas, lung, and prostate cancer. However, it is important to note that the best results are achieved from severe calorie restriction (<1,000 calories per day). If you are considering using calorie restriction along with your cancer treatment, make sure you consult your cancer care team first.
Vitamin D
Vitamin D has been found to offer cancer protection in several ways:
- Regulating genetic expression
- Increasing the self-destruction of mutated cells
- Reducing the spread of reproduction of cancer cells
- Causing cells to become differentiated
- Reducing the growth of new blood vessels from pre-existing ones
Vitamin D deficiency plays a crucial role in cancer development. So, to decrease your risk of cancer, you must optimize your vitamin D levels with sun exposure. If you are being treated for cancer, you will need even higher levels of Vitamin D.
To maximize your vitamin D levels, it is best to get in the sun as much as possible, but not so much that you burn your skin. Ideally, you want to have 40% or more of your skin exposed to the sun for at least 15 minutes per day in the early afternoon. If you can’t get that much sun exposure every day, then supplementing with around 4,000 IU of vitamin D3 daily may be your next best option.
Omega 3s
Omega 3s have been found to have many properties that can help with cancer treatment. Here are some examples of what these fatty acids can do:
- Reduce the cachexia phenotype. One study has shown that omega-3 fatty acids can decrease the degree of weakness and weight loss that cancer patients may experience as a result of the disease.
- Helps improve overall metabolic health. Omega 3s can help lower blood sugar and inflammation levels, which helps decrease the likelihood that genes will mutate and create cancerous cells.
If you’d like to see more information on omega 3s, check out this article.
EGCG
Recent studies suggest that the green tea polyphenol (EGCG) can target glutamine metabolism. In other words, any cancer that is able to sustain itself with glutamine may become inhibited by what EGCG does to prevent glutamine metabolism.
EGCG and other glutamine-targeting strategies could be even more effective when combined with energy restricting diets. This would provide us with a non-toxic way of targeting both glucose and glutamine metabolism in an attempt to limit the ability of resilient cancer cells to fuel themselves.
Sleep
Sleep is a threefold factor. First, you must get enough sleep. Second, you need to get continuous sleep. And finally, you need to sleep between certain hours for optimal effectiveness of your sleep time.
Research has confirmed that sleep between the hours of 10 pm and 6 am is optimal as certain hormonal fluctuations occur through the day and night, and if you are engaging in the right activities during those times, you are able to get the most out of your hormones.
For example, there is a spike in melatonin that occurs between midnight and 1 am that actually decreases the estrogen your body is producing and boosts your immune system.
Conversely, other studies have shown that going to sleep after 10 could significantly increase your risk of breast cancer and having disrupted sleep patterns can speed up cancer growth. So, make sure you prioritize sleep!
Stress Regulation
Stress causes major issues within our bodies. It depresses the immune system, and it also raises the risk of certain cancers. However, avoiding stress completely is also unhealthy.
The best way to regulate stress is by making sure it doesn’t stick around for too long. If you are obsessively thinking about something that stresses you out or your life is constantly triggering a stress response, then you need to take the necessary steps to decrease your stress levels.
Some stress relieving methods that may work for you are a midday walk, a hike, sunbathing, a quick puzzle game, a breath of fresh air, yoga, meditation, therapy, etc. Whatever helps you reduce your stress, add it to your daily schedule.
Exercise
Exercise reduces insulin and IGF-1 levels, which discourages the growth and spread of the cancer cells. (Sounds a lot like the ketogenic diet, right? Imagine the benefits of doing BOTH!)
Also, exercise helps boost the circulation of immune cells throughout your body – those little protector cells of ours that defend us from everything from a minor cold to life-threatening things like cancer.
The combination of exercise and intermittent fasting has been shown to significantly reduce your cancer risk and stimulate healing processes throughout your body as well. So, imagine what a ketogenic diet, exercise, and intermittent fasting could do in your healing journey.
Putting It All Together — A Comprehensive Approach to Cancer
Our ability to treat various forms of cancer has been steadily improving and will likely continue to improve. However, there will not be any sudden “cure for cancer” — the disease itself is too diverse and complex for it to have a universal cure.
The best treatment approach for cancer includes addressing it as early as possible with standard cancer treatments and dietary and lifestyle modification.
Some scientifically proven strategies that you can use along with standard cancer treatment and/or to prevent cancer growth are:
- Following a ketogenic diet.
- Using intermittent fasting and/or multiple day fasts
- Implementing a calorie restricted diet (like a keto diet that is geared toward weight loss)
- Supplementing with Vitamin D3, sunlight, omega 3s, and EGCG.
- Getting an adequate amount of sleep.
- Using stress relieving strategies.
- Increasing your activity levels.
In fact, you will most likely get the best results by adding all seven of these recommendations to your cancer treatment plan. Just make sure you consult your physician before implementing these suggestions.
Sources
- Cancer Deaths Continue to Decline — Science-Based Medicine
- The Development and Causes of Cancer — NCBI Bookshelf
- Cancer as a metabolic disease — Nutrition & Metabolism
- The hallmarks of cancer. — NCBI
- Obesity and Cancer — National Cancer Institute
- Energy Deregulation: Licensing Tumors to Grow — Science
- The Warburg Effect: How Does it Benefit Cancer Cells? — NCBI
- Classifying the evolutionary and ecological features of neoplasms — NCBI
- Natural Selection in Cancer Biology: From Molecular Snowflakes to Trait Hallmarks — NCBI
- Body fatness as a cause of cancer: epidemiologic clues to biologic mechanisms — Society for Endocrinology
- Ketogenic diet does not “beat chemo for almost all cancers” — Science-Based Medicine
- Ketones and lactate “fuel” tumor growth and metastasis — Cell Cycle
- Fructose Induces Transketolase Flux to Promote Pancreatic Cancer Growth — Cancer Research
- Glutamine and cancer: cell biology, physiology, and clinical opportunities — The Journal of Clinical Investigation
- Links between metabolism and cancer — Genes & Development
- mTOR Complex1–S6K1 signaling: at the crossroads of obesity, diabetes and cancer — Elsevier
- Fasting and cancer treatment in humans: A case series report — NCBI
- Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. — NCBI
- Vitamin D and Cancer Prevention — National Cancer Institute
- Disrupted sleep speeds up cancer — Medical News Today
- Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. — Medscape
- Night Work and Risk of Breast Cancer — Epidemiology
- Chemicals in Meat Cooked at High Temperatures and Cancer Risk — National Cancer Institute
- Light at Night, Chronodisruptions, Melatonin Suppression, and Cancer Risk: A Review. — Critical Reviews in Oncogenesis
- Is there a role for carbohydrate restriction in the treatment and prevention of cancer? — NCBI
- Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. — Journal of the American College of Nutrition
- Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. — NCBI
- Pilot trials in clinical research: of what value are they? — NCBI
- Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. — NCBI
- Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. — NCBI
- Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. — NCBI
- Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism — NCBI
- Effect of Low-Intensity Aerobic Exercise on Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-Binding Proteins in Healthy Men — Hindawi
- Benefits of Sunlight: A Bright Spot for Human Health — NCBI
- Phenotypes and genetic markers of cancer cachexia — era