We recently touched on how you can use the ketogenic diet to control symptoms of diabetes such as elevated glucose and triglycerides. In this article, we examine research showing the impact that the ketogenic diet has on levels of the hormone insulin, a key regulator of blood sugar in the body.
What is Insulin’s Role in the Body?
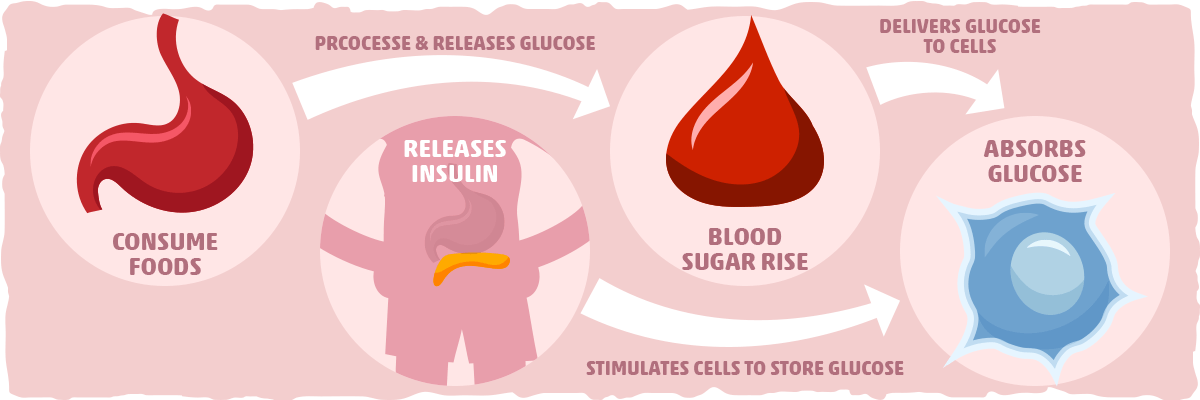
Before we look at the research, we need to know our main players. Insulin is a protein-based hormone produced by beta-cells located in the pancreas. The pancreas, which is located under the stomach, also produces enzymes that aid with digestion.
Insulin’s primary purpose is to regulate the metabolism of fats and carbohydrates. The digestive system breaks down carbohydrates, such as sugars and starches, into a molecule called glucose. This compound can be used by cells to produce energy through a process called cellular respiration. Insulin allows cells in the body absorb glucose, ultimately lowering levels of glucose in the blood stream.
After a meal is consumed, blood glucose levels increase and the pancreas responds by releasing insulin into the blood. Insulin assists fat, liver, and muscle cells absorb glucose from the blood, resulting in lower levels of blood glucose. Insulin stimulates liver and muscle tissues to store excess glucose as a molecule called glycogen and also reduces glucose production by the liver. When blood sugar is low, the hormone glucagon (produced by alpha-cells in the pancreas) stimulate cells to break down glycogen into glucose that is subsequently released into the blood stream.
In healthy people who do not have type II, these functions allow levels of blood glucose and insulin to stay in a normal range.
What Is Insulin Resistance and Why Is It a Problem?
Unfortunately, for many Americans and other people throughout the world, levels of glucose and insulin are imbalanced. Insulin resistance is a condition in which cells throughout the body no longer respond to the normal actions of the hormone insulin. More specifically, muscle, liver, and fat cells have difficulty absorbing glucose from the bloodstream. In order to compensate for this, the body produces more insulin.
Initially, the body is typically able to overcome the insulin resistance and levels of blood glucose stay in a healthy range. However, as resistance builds up, the beta cells in the pancreas are unable to produce sufficient insulin to regulate blood glucose. As a result, blood glucose levels can elevate and result in prediabetes, diabetes, and other chronic health disorders such as metabolic syndrome. [1]
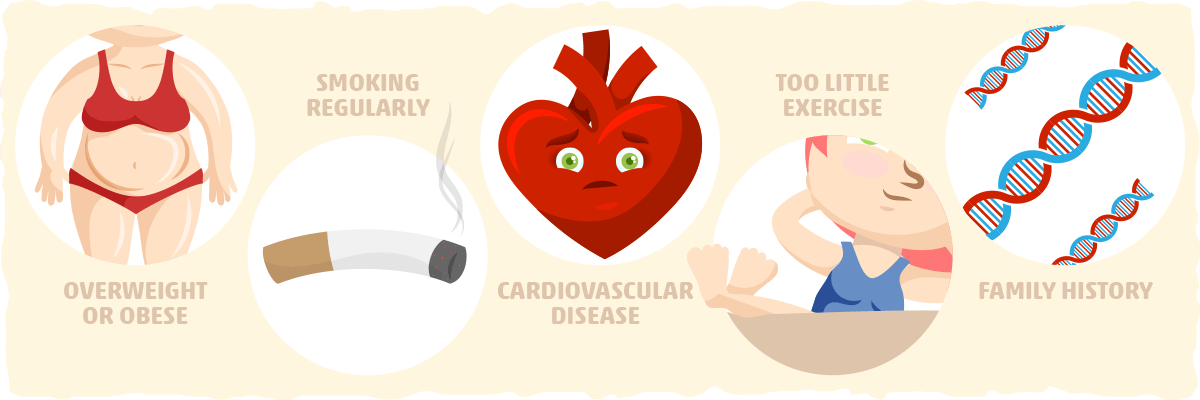
Insulin resistance is very common. An estimated 24% of US adults aged 20 years or older have the condition. [2] Interestingly, many people with insulin resistance are unaware they have it until they develop type 2 diabetes or another serious, chronic condition. That is why it is crucial to get screened every three years if you are 45 years or older or have a risk condition that puts you at increased risk for insulin resistance. [3] Additionally, it is important to get tested if you have one of the following conditions that put you at risk:
- Being overweight or obese
- Smoking regularly
- A waist measurement over 40 in for men and 35 in for women
- Having cardiovascular disease
- Exercising minimally
- Having familial history of diabetes
- Being of Hispanic, African-American, American Indian, Pacific Islander, or Asian American origin
- Having high blood pressure of 140/90 mmHg or above
- HDL cholesterol levels below 35 mg/dL and/or triglyceride levels above 250 mg/dL
- Having polycystic ovary syndrome (PCOS)
For a complete list of risk factors, visit the National Institute of Diabetes and Digestive and Kidney Diseases.
How to Treat Insulin Resistance
Given the prevalence of insulin resistance, how can one treat this common condition? The FDA hasn’t approved of any drugs to specifically treat insulin resistance or pre-diabetes. However, physicians may prescribe two classes of drugs normally used to treat type 2 diabetes- biguanides and thiazolidinediones- which sensitize muscle cells, liver cells, and other tissues to the effects of insulin.
That being said, the best way to treat insulin resistance is to modify one’s lifestyle behaviors. Weight-loss and exercise are considered to be the most effective methods in restoring the ability of tissues to properly respond to insulin. [3] Interestingly, smoking contributes to insulin resistance, so many physician recommend reducing or ceasing smoking altogether. [4] In order to reduce insulin secretion, dietitians also recommend lowering carbohydrate intake. [3]
Provided that the ketogenic diet is an effective method in losing weight and lowering blood glucose, can it be utilized to lower insulin resistance and increase levels of insulin in the body? Below we document the in-depth research.
Landmark Study: Ketogenic Diet Impact on Insulin Levels
The first study assessing the direct impact of ketogenic diets on insulin resistance was conducted ten years ago in 2005. In it, researchers recruited 10 obese patients with type 2 diabetes and had them consume their normal diets for 1 full week. Then, the researchers provided them with a high-fat ketogenic diet for 2 weeks.
After the intervention, the 10 subjects experienced a mean energy intake decrease from 3111 kcal/day to 2164 kcal/day- representing a 30.4% decrease. This resulted in a weight-loss of 1.65 kg. More significantly, insulin sensitivity improved by approximately 75%- a dramatic increase. [5] Additionally, hemoglobin A1c levels decreased from 7.3% to 6.8%, mean triglyceride levels decreased by 35% and cholesterol decreased by 10%. [5]
Key Takeaways: A ketogenic diet may substantially increase insulin sensitivity in obese subjects with type 2 diabetes.
Recommendation: If you are trying to increase your insulin sensitivity, consider utilizing a ketogenic diet.
Side by Side Comparison: Ketogenic vs. Moderate Fat Diet
The next study examining the impact of the ketogenic diet on insulin resistance was conducted in 2006. Researchers recruited 83 subjects with an average age of 48 years, total cholesterol of 5.9 mmol/L, and BMI of 33. The subjects were divided into three groups and asked to eat one of three equal-calorie diets. The first group consumed a very low fat diet (VLF) consisting of 70% carbohydrates, 10% fat (3% saturated fat), and 20% protein.
The second group consumed a diet high in unsaturated fat (HUF) consisting of 50% carbohydrates, 30% fat (6% saturated fat), and 20% protein. The final diet mimicking the ketogenic diet, called the very low carbohydrate diet (VLCARB), consisted of 4% carbohydrates, 61% fats (20% saturated fat), and 35% protein. All subjects eat one of these equal-calorie diets for 8 weeks followed by a 4 week period in which they consumed the same diet.
After the intervention, the researchers noted that each diet led to similar reductions in weight-loss and fat loss composition. However, the VLCARB diet lowered fasting insulin by 33% and the HUF diet lowered it by 19%. [6] The VLF diet had no impact on fasting insulin levels. Additionally, the “the VLCARB meal also provoked significantly lower post prandial glucose and insulin responses than the VLF and HUF meals.” [6] Additionally, the VLCARB diet lowered triglycerides more than the other two diets. Because of these collective findings, the researchers suggested that “VLCARB may be useful in the short-term management of subjects with insulin resistance and hypertriacylglycerolemia.” [6]
From Noakes et. al
Another study in 2009 sought to compare the effects of ketogenic and low fat diets on insulin resistance in overweight, healthy subjects. In a randomized, controlled, calorie-restricted dietary intervention lasting 12 weeks, researchers recruited 40 male and female subjects aged 18-55 years. They had a BMI of greater than 25 kg/m2 and had at high levels of triglycerides (>150 mg/dL). However, they had no metabolic, endocrine, or glucose-related disorders and did not take any medications or supplements to deal with these conditions.
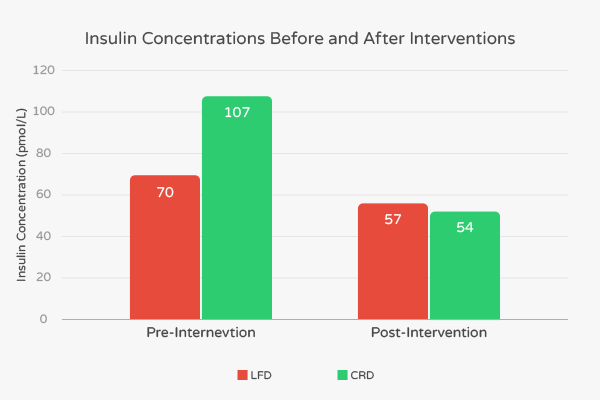
The subjects were divided into two groups. The first group of 20 consumed a carbohydrate-restricted diet (CRD) which consisted of 12% calories from carbohydrates, 59% from fat, and 29% protein. Before the intervention, these subjects had an average BMI of 33.5 kg/m2, percent body fat of 40.6%, and age of 32.6 years. The second group of 20 ate low-fat diet (LFD) consisting of 56% carbohydrate, 24% fat, and 20% protein. Before the intervention, these subjects had an average BMI of 32.1, percent body fat of 39.0%, and age of 36.9 years old.
At the end of the intervention, researchers noted favorable changes in BMI, body fat percentage, levels of triglycerides and LDL in both the LFD and CRD groups. However, these decreases were all more pronounced in the CRD group. Fasting insulin concentration in the LFD group decreased from 70 pmol/L to 57 pmol/L, representing an 18.6% decrease. [7] In contrast, the fasting insulin concentration in the CRD group decreased from 107 pmol/L to 54 pmol/L, representing a 49.5% decrease. [7]
Also, according to the authors, “fat mass in the abdominal region, associated with many features of the insulin resistance syndrome, was similarly decreased significantly more in subjects consuming the CRD than subjects following the LFD (-828 g vs -506 g).” [7] These results suggest that low-carbohydrate ketogenic diets confer additional benefits to people with high levels of insulin.
Key Takeaways: Low-carbohydrate ketogenic diets decrease fasting insulin levels compared to moderate and low fat diets in overweight subjects with elevated levels of lipids who are otherwise healthy.
Recommendations: If you have high levels of insulin or insulin resistance, consider using a low-carbohydrate ketogenic diet with a moderate saturated fat content.
Fasting Insulin in an Obese, Ethnically-diverse Population after Ketogenic Diet Intervention
Given the previous study’s short duration, how does the ketogenic diet affect insulin resistance and other key metrics of diabetes in the long-term compared to alternative diets?
In 2010, researchers recruited 146 overweight and obese patients from the Department of Veterans Affairs in Durham, North Carolina. The mean age of these subjects was 52 years and the mean BMI was 39.3. Approximately 72% were men, 55% were African-American, and 32% had type 2 diabetes. The researchers placed the subjects in one of two weight-loss interventions that lasted 48 weeks.
The first group of 74 participated in a low-fat diet which had a 500-1000 kilocalorie/day deficit and less than 30% of calories from fat. Additionally, these subjects consumed three 120 mg tablets of the weight-loss orlistat drug per day. The second group of 72 ate a low-calorie ketogenic diet (LCKD) which initially had fewer than 20 grams of carbohydrates per day.
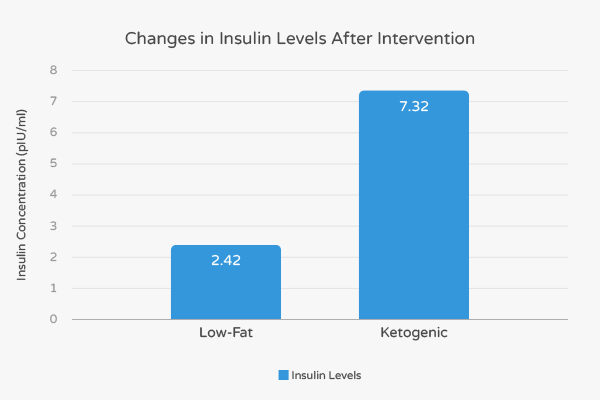
Overall, 52 out of 72 subjects in the LCKD group (79%) and 65 of the low-fat group (88%) completed the 48-week long trials. Both groups experienced similar levels of weight-loss and had similar levels of improvement in regards to heart-healthy high-density lipoprotein cholesterol (HDL-C) and triglycerides. However, only the LCKD group experienced improvements in levels of insulin, glucose, and hemoglobin A1C.
More specifically, the fasting insulin levels in the LCKD group decreased by 7.32 pIU/mL and 2.42 pIU/mL in the low-fat group. [8] Thus, fasting insulin levels in the LCKD subjects decreased by over three times compared to the low-fat subjects. Diabetes patients who completed the LCKD intervention experienced a 13.49 pIU/mL decrease in insulin while those that completed the low-fat intervention experienced an 8.35 pIU/mL decrease in insulin. [8] Collectively, these findings suggest that a LCKD diet has a stronger impact on insulin levels compared to low-fat diets supplements by drugs.
Key Takeaways: Low carb ketogenic diets have a greater impact on metrics related to diabetes, such as fasting insulin levels, in obese and overweight individuals when compared to standard low-fat/drug weight-loss interventions.
Recommendations: If you are diabetic with high levels of fasting insulin, consider utilizing a ketogenic diet.
Impact of Ketogenic Diet Impact on Fasting Insulin in Obese Children
In 2012, researchers sought to evaluate the effects of the ketogenic diet in obese children. For their study, they recruited 58 subjects and placed them in one of two different diets for 6 months. The first group consumed a carbohydrate-restricted diet while the second group ate a hypocaloric diet.
After the intervention, both groups had significantly reduced their body weight, body fat, waist circumference, and fasting insulin. However, these differences were more pronounced in the ketogenic group. At the conclusion of their study, the researchers stated that “the ketogenic diet revealed more pronounced improvements in weight loss and metabolic parameters than the hypocaloric diet and may be a feasible and safe alternative for children’s weight loss.” [9]
Key Takeaways: Ketogenic diet may help with insulin resistance in obese children.
The Impact of Carbohydrate Restriction on Insulin in Healthy Athletes
Most studies examining the impact of the ketogenic diet on insulin resistance have used middle-aged, overweight subjects. However, what impact does the ketogenic diet have on insulin resistance on younger, healthy people? In a 2014 study, researchers sought to shed light on that question and recruited 8 male subjects with a median age of 28.3 years. They had a mean BMI of 24.93- making the subjects, on average, normal-weight. Additionally, all subjects had participated in off-road cycling for at least 5 years and had a minimal VO2max of 55/ml/kg- making them a very fit group.
The intervention consisted of two separate phases where the subjects ate an experimental diet and then performed a three-day, moderate-to-high volume cycling training protocol where blood samples were taken. The first testing phase lasted three days and was preceded by four weeks of a mixed diet. This diet consisted of 50% calories from carbohydrates, 30% from fats, and 20% from protein. The second testing phase lasted three days and was preceded by a ketogenic diet composed of 70% fat, 15% protein, and 15% carbohydrates.
The first two days of the cycling protocol consisted of a moderate-intensity exercise regimen. On the third day, each cyclist performed a continuous effort with progressively increasing intensity for a total of 105 minutes. For the first 90 minutes of the test, the subjects performed exercise set at 85% of the intensity as measured in watts. The last 15 minutes of the continuous effort were conducted at maximum individual intensity.
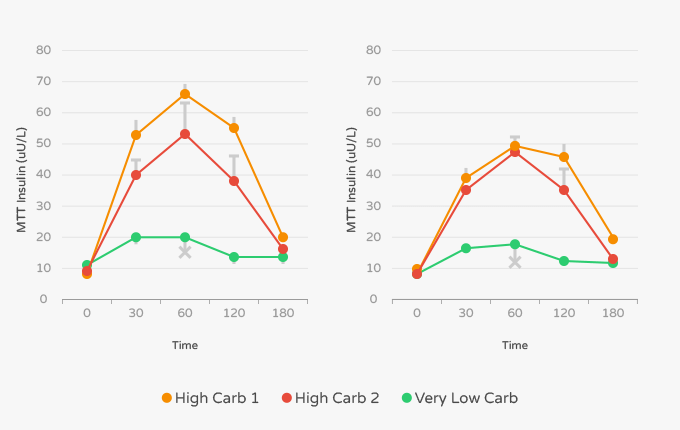
The researchers measured insulin levels during each exercise protocol after each intervention. After the mixed diet, the authors noted that insulin levels were 19.21 U/L at rest, 6.02 U/L 45 minutes in, 5.45 U/L after 90 minutes, and 9.89 U/L during maximum effort. [10] In contrast, insulin levels during the exercise protocol after the ketogenic diet were 6.02 U/L at rest, 4.25 U/L after 45 minutes, 4.97 U/L after 90 minutes, and 5.63 U/L after maximum effort. [10]
The authors of the study also stated that the “high volume training on a ketogenic diet increases fat metabolism during exercise, reduces body mass and fat content and decreases post exercise muscle damage.” [10] Collectively, these findings suggest that the ketogenic diet can benefit insulin resistance and recovery in athletes.
Key Takeaways: Low-carbohydrate diets help lower insulin levels in the body and may help with athletic performance.
Recommendation: If you are an endurance athlete concerned about insulin resistance or elevated glucose levels, considering using the ketogenic diet.
Supplementation & Ketogenic Diet: A Winning Combination for Insulin?
Provided the ketogenic diet’s positive impact on insulin resistance, can it be modified to confer even greater benefits?
In a study from earlier this year, researchers recruited 35 overweight subjects between 25 and 65 years old and divided them into two groups. The first group of 18 were instructed to follow a Mediterranean ketogenic diet for four weeks. They had an average age of 56.3 years and mean BMI of 29.34. Researchers instructed the second group of 17 subjects to follow the same ketogenic diet and also take three fish-oil supplements containing omega-3 fatty acids per day. These molecules are usually found in fatty fish, such as sardines and mackerel, and also in walnuts and chia seeds. These subjects had an average age of 58.1 years and a mean BMI of 29.17.
At the conclusion of the study, both groups showed a significant and similar decrease in BMI and per cent fat. Additionally, both showed a decrease in total cholesterol and low-density lipoprotein (LDL) but no significant change in HDL-cholesterol. These findings are in-line with many other research studies on the ketogenic diet.
The result for insulin proved particularly interesting. The subjects who consumed the ketogenic diet alone went from an average insulin concentration of 9.3 nIU/mL to an average insulin concentration of 7.4 nIU/mL of insulin. [11] This represents a 20.4% average decrease. In contrast, the subjects who consumed the ketogenic diet along with omega-3 fatty acid supplementation decreased their average insulin concentration from 11.1 nIU/mL to 6.3 nIU/mL. [11] This represents a 43% decrease in average insulin concentration- more than twice the decrease seen in the ketogenic diet alone. Additionally, subjects who took omega-3 fatty acid supplements experienced greater decreases in triglycerides and inflammation markers such as adiponectin and interleukin 1 beta (IL-1B). [11]
As a result of their findings, “the authors concluded that [omega-3] supplementation improved the positive effects of a ketogenic Mediterranean diet with phytoextracts on some cardiovascular/metabolic risk factors and inflammatory state.” [11]
Key Takeaways: Taking supplements of omega-3 fatty acids while on the ketogenic diet may confer additional benefits on metrics related to diabetes such as insulin resistance and levels of blood triglycerides and inflammatory markers in healthy, overweight subjects. However, these supplements do not affect other metrics related to diabetes such as fasting glucose.
Recommendations: If you have high levels of insulin, inflammation, or triglycerides are high, consider adding regular omega-3 fatty acid supplements (such as fish oil capsules) to your ketogenic diet.
Why the Keto Diet Has the Potential to Reverse Insulin Resistance
The majority of human studies on how the keto diet impacts blood sugar control share a similar theme: a low-carb, high-fat diet has the potential to reverse insulin resistance.
However, we have yet to explore the mechanisms behind why keto is so effective in this case. By understanding the “why” behind the research results, we can extract practical strategies that may help us reverse insulin resistance from a dietary standpoint.
Although there is currently no “gold standard” dietary treatment for insulin resistance, we do have promising evidence that supports the effectiveness of these four factors:
1. Calorie Restriction
Most of the research on type 2 diabetes and insulin resistance supports the use of calorie restriction for improving many of the metabolic issues that contribute to these conditions. [12] [13] Both the keto diet and low-calorie diets have been shown to help reduce insulin resistance, and many researchers postulate that being in a calorie deficit is the key variable behind these positive effects.[12] [13]
At first, this may seem a bit peculiar. Why would a calorie deficit be so helpful when it is the carbs that provoke a significant insulin response?
To answer this question, let’s take a look at what happens in the body from a broader perspective. When we consume an excess amount of calories, our body is flooded with various energy sources. This tends to increase energy use, fat storage, and inflammatory molecule production. In the short term (i.e., after a high-calorie meal), the body has no issue handling the influx of energy. [14]
However, when your diet and lifestyle consistently puts you into a state of energy surplus, your cells will respond by becoming resistant to insulin. This happens because your cells are not designed to handle a sustained excess of energy.
In fact, if they continue to let more energy in, they will accumulate inflammatory molecules that can induce metabolic dysfunction and apoptosis (cell death). To avoid cellular disaster, the cells will start to ignore insulin’s message, causing blood sugar levels and insulin levels to increase further. [14]
One way to reduce the insulin resistance and increase insulin sensitivity is by putting your cells in an “underfed” or energy deficient state. Arguably, the most effective way to do this is by maintaining a calorie deficit. [14]
Although eating fewer calories is the simplest approach to reducing insulin resistance, it is not easy for many of us to implement successfully. This is where the keto diet truly shines.
By eating more fat and restricting carbs, we tend to increase overall satiety which leads to a spontaneous reduction in calorie intake. [15] For many of us, the keto diet provokes such a filling response that maintaining a calorie deficit and losing weight becomes both simple and easy.
In other words, the keto diet will help send our cells into an “underfed” state, giving them a potent stimulus to reduce their insulin resistance.
Along with this, keto also helps us lose fat, decrease our insulin response to meals, and reap the benefits of ketones. Let’s take a look at each one of these factors and how they affect insulin resistance next.
2. Body Fat Reduction
Although calorie restriction directly decreases insulin resistance by reducing the energy load on our cells, it also improves insulin signaling indirectly by reducing body fat. According to the current research literature, both visceral and subcutaneous fat play a role in insulin resistance. [16]
Visceral fat — the type of fat that accumulates in our abdominal region, around our vital organs — is not a passive storage depot for energy, like it was widely believed to be. In fact, this insidious fat has been found to act as an endocrine organ, secreting various signaling molecules that can, directly and indirectly, increase insulin resistance. [17]
By reducing our visceral fat stores, we can decrease the secretion of these potentially harmful signaling molecules and improve overall metabolic health. Altogether, this will help increase insulin sensitivity.
Subcutaneous fat, on the other hand, tends to be relatively inert compared to visceral, functioning as a storage depot for excess energy. You will typically find this fat type accumulating on the thighs, arms, chest, and/or lower abdomen, just underneath the skin.
In healthy individuals, subcutaneous fat predominantly acts as an alternative energy source and insulation. However, in people who chronically over-consume calories, their subcutaneous fat cells may start to increase inflammation levels and disrupt insulin signaling as a result of getting too big and becoming dysfunctional. [17]
To keep our fat cells from fighting against us, we must adopt a healthier lifestyle that prevents us from being in a calorie surplus and gaining weight. The keto diet is one lifestyle change that has been proven time and time again to help us lose fat and improve metabolic health.
3. Carbohydrate Restriction
To help diabetics manage their blood sugar levels, doctors will typically prescribe a diet that mostly consists of low glycemic index foods. [18] In other words, they will advise against eating foods that cause significant increases in blood sugar levels as a way to help the patient regulate his or her blood sugar levels without needing so much medication.
One way of decreasing the glycemic index of your diet (i.e., the degree to which it increases your blood sugar levels) is by limiting carbs altogether. Since carbs are the only macronutrient that can cause significant fluctuations in blood sugar levels, restricting them will reduce your insulin needs and potentially increase insulin sensitivity. [18]
By following a low carb diet, for example, insulin resistant individuals can bring their insulin, blood sugar, and insulin sensitivity back to healthier levels.[19] [20] In fact, the keto diet has been found to improve glycemic control more effectively than the low glycemic index diet. [21]
4. Ketones
By restricting carbs to the degree that the keto diet recommends, you will stimulate the production of ketones and eventually enter nutritional ketosis. As a result, you will start burning more ketones as a way to make up for the lack of dietary carbohydrates.
Ketones also play a crucial role as signaling molecules that stimulate specific genetic processes related to metabolic health and longevity. [22] For example, our primary ketone body, beta-hydroxybutyrate (BHB), has been found to inhibit some histone deacetylases (HDACs) in a way that may lower glucose and insulin levels, decrease insulin resistance, prevent weight gain, and improve energy efficiency. [22]
Unfortunately, most of the research on the signaling properties of ketones has been done on mice, so it is unclear if ketones will do the same for humans. With that being said, ketones have been found to be an appetite suppressant in humans. [23] This means that they can indirectly improve insulin sensitivity by causing us to eat fewer calories.
Putting It All Together — Keto as a Treatment for Insulin Resistance
Insulin resistance is a widespread problem that, if not properly managed, can lead to prediabetes and eventually type II diabetes. Thankfully, abundant research suggests that modifying one’s diet to a low-carbohydrate or ketogenic diet can help people lower their insulin to healthy levels and may even help reverse insulin resistance. Nearly all comparative studies on obese subjects suggest that low-carbohydrate diets are superior to low-fat diets in optimizing levels of insulin.
Altogether, these promising results can be explained by the fact that the keto diet:
- helps us sustain a calorie deficit, which increases insulin sensitivity.
- helps us lose fat, which reduces inflammation and the production of signaling molecules that provoke insulin resistance.
- significantly reduces our glycemic load, which helps us regulate blood sugar levels and decrease our insulin needs.
- stimulates the production and usage of ketones. This can, directly and indirectly, reduce insulin resistance via multiple mechanisms.
Throughout this article, we looked at the data and the principles behind insulin resistance and the keto diet, but we never addressed what you can do about it directly. If you’d like to learn about practical strategies that may help you reverse insulin resistance and improve your blood sugar levels, I recommend reading these articles:
- Insulin Sensitivity: How You Can Optimize It for Better Health
- How to Lower Your Blood Sugar Naturally
Sources
- DeFronzo, Ralph A., and Eleuterio Ferrannini. “Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease.” Diabetes care 14.3 (1991): 173-194.
- Meigs, James B. “Epidemiology of the insulin resistance syndrome.” Current diabetes reports 3.1 (2003): 73-79.
- McAuley, Kirsten A., et al. “Diagnosing insulin resistance in the general population.” Diabetes care 24.3 (2001): 460-464.
- Bajaj, Mandeep. “Nicotine and insulin resistance: when the smoke clears.”Diabetes 61.12 (2012): 3078-3080.
- Boden, Guenther, et al. “Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes.” Annals of internal medicine 142.6 (2005): 403-411.
- Noakes, Manny, et al. “Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk.” Nutrition & metabolism 3.1 (2006): 7.
- Volek, Jeff S., et al. “Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet.” Lipids 44.4 (2009): 297-309.
- Yancy, William S., et al. “A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss.” Archives of internal medicine 170.2 (2010): 136-145.
- Partsalaki, Ioanna, Alexia Karvela, and Bessie E. Spiliotis. “Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents.” Journal of Pediatric Endocrinology and Metabolism 25.7-8 (2012): 697-704.
- Zajac, Adam, et al. “The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists.” Nutrients 6.7 (2014): 2493-2508.
- Paoli, Antonio, et al. “Effects of n-3 Polyunsaturated Fatty Acids (ω-3) Supplementation on Some Cardiovascular Risk Factors with a Ketogenic Mediterranean Diet.” Marine drugs 13.2 (2015): 996-1009.
- Clamp, Hume, et al. “Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial.” The Lancet 391.10120 (2017): 541-551.
- Clamp, Hume, et al. “Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history.” Nutrition & Diabetes 7 (2017): 282.
- Masterjohn, et al. “Insulin Resistance Isn’t All About Carbs and Insulin.” Chris Masterjohn, PhD, 22 Feb. 2017, chrismasterjohnphd.com/2016/08/24/insulin-resistance-isnt-all-about-carbs-and-insulin/.
- “How to Lose Weight on a Ketogenic Diet.” Ruled Me, 17 Feb. 2018, www.ruled.me/how-to-lose-weight-ketogenic-diet/.
- Patel, Pavankumar, and Nicola Abate. “Body Fat Distribution and Insulin Resistance.” Nutrients 5.6 (2013): 2019–2027.
- Coelho, Marisa, Teresa Oliveira, and Ruben Fernandes. “Biochemistry of Adipose Tissue: An Endocrine Organ.” Archives of Medical Science : AMS 9.2 (2013): 191–200.
- “Glycemic Index and Diabetes.” American Diabetes Association, www.diabetes.org/food-and-fitness/food/what-can-i-eat/understanding-carbohydrates/glycemic-index-and-diabetes.html.
- Krebs, J D, et al. “Improvements in Glucose Metabolism and Insulin Sensitivity with a Low-Carbohydrate Diet in Obese Patients with Type 2 Diabetes.” Advances in Pediatrics.
- Yancy, William S et al. “A Low-Carbohydrate, Ketogenic Diet to Treat Type 2 Diabetes.” Nutrition & Metabolism 2 (2005): 34.
- Westman, Eric C et al. “The Effect of a Low-Carbohydrate, Ketogenic Diet versus a Low-Glycemic Index Diet on Glycemic Control in Type 2 Diabetes Mellitus.” Nutrition & Metabolism 5 (2008): 36.
- Newman, John C., and Eric Verdin. “Ketone Bodies as Signaling Metabolites.” Trends in endocrinology and metabolism: TEM 25.1 (2014): 42–52.
- Stubbs, Brianna J. et al. “A Ketone Ester Drink Lowers Human Ghrelin and Appetite.” Obesity